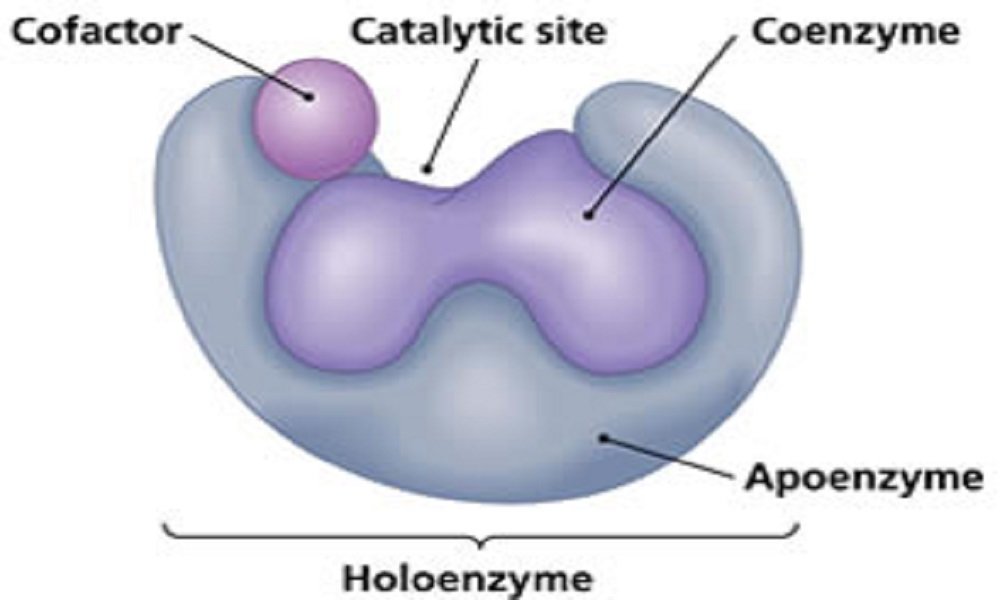
- Enzymes may be simple proteins or complex enzymes. A complex enzyme contains a non-protein part, called a prosthetic group (co-enzymes). Coenzymes are heat stable low molecular weight organic compound.
- The combined form of protein and the co-enzyme are called as holo-enzyme. The heat labile or unstable part of the holo-enzyme is called as apo-enzyme. The apo-enzyme gives necessary three-dimensional structures required for the enzymatic chemical reaction.
- Co-enzymes are very essential for the biological activities of the enzyme. Co-enzymes combine loosely with apo-enzyme and are released easily by dialysis. Most of the co-enzymes are derivatives of vitamin B complex group of substance.
- One molecule of the co-enzyme with its enzyme is sufficient to convert a large group of the substrate.
- Co-enzymes are further divided into two groups. The first groups of co-enzymes are a part of reaction catalyzed by oxidoreductase by donating or accepting hydrogen atoms or electrons. The first group of co-enzymes are also called as co-substrates or secondary substrates. Because they are involved in counterbalance in change occurring in the substrate. The second group of co-enzymes involves in reactions transferring groups other than hydrogen.
Role of Coenzymes
- The functional role of coenzymes is to act as transporters of chemical groups from one reactant to another. The chemical groups carried can be as simple as the hydride ion (H+ + 2e) carried by NAD or the mole of hydrogen carried by FAD; or they can be even more complex than the amine (-NH2) carried by pyridoxal phosphate.
- Since coenzymes are chemically changed as a consequence of enzyme action, it is often useful to consider coenzymes to be a special class of substrates, or second substrates, which are common to many different holoenzymes.
- In all cases, the coenzymes donate the carried chemical grouping to an acceptor molecule and are thus regenerated to their original form. This regeneration of coenzyme and holoenzyme fulfils the definition of an enzyme as a chemical catalyst since (unlike the usual substrates, which are used up during the course of a reaction) coenzymes are generally regenerated.
Examples of Coenzymes
Most organisms cannot produce coenzymes naturally in large enough quantities to be effective. Instead, they are introduced to an organism in two ways:
Vitamins
Many coenzymes, though not all, are vitamins or derived from vitamins. If vitamin intake is too low, then an organism will not have the coenzymes needed to catalyze reactions. Water-soluble vitamins, which include all B complex vitamins and vitamin C, lead to the production of coenzymes. Two of the most important and widespread vitamin-derived coenzymes are nicotinamide adenine dinucleotide (NAD) and coenzyme A.
NAD is derived from vitamin B3 and functions as one of the most important coenzymes in a cell when turned into its two alternate forms. When NAD loses an electron, the low energy coenzyme called NAD+ is formed. When NAD gains an electron, a high-energy coenzyme called NADH is formed.
NAD+ primarily transfers electrons needed for redox reactions, especially those involved in parts of the citric acid cycle (TAC). TAC results in other coenzymes, such as ATP. If an organism has a NAD+ deficiency, then mitochondria become less functional and provide less energy for cell functions.
When NAD+ gains electrons through a redox reaction, NADH is formed. NADH, often called coenzyme 1, has numerous functions. In fact, it is considered the number one coenzyme in the human body because it is necessary for so many different things. This coenzyme primarily carries electrons for reactions and produces energy from food. For example, the electron transport chaincan only begin with the delivery of electrons from NADH. A lack of NADH causes energy deficits in cells, resulting in widespread fatigue. Additionally, this coenzyme is recognized as the most powerful biological antioxidant for protecting cells against harmful or damaging substances.
Coenzyme A, also known as acetyl-CoA, naturally derives from vitamin B5. This coenzyme has several different functions. First, it is responsible for initiating fatty acid production within cells. Fatty acids form the phospholipid bilayer that comprises the cell membrane, a feature necessary for life. Coenzyme A also initiates the citric acid cycle, resulting in the production of ATP.
Non-Vitamins
Non-vitamin coenzymes typically aid in chemical transfer for enzymes. They ensure physiological functions, like blood clotting and metabolism, occur in an organism. These coenzymes can be produced from nucleotides such as adenosine, uracil, guanine, or inosine.
Adenosine triphosphate (ATP) is an example of an essential non-vitamin coenzyme. In fact, it is the most widely distributed coenzyme in the human body. It transports substances and supplies energy needed for necessary chemical reactions and muscle contraction. To do this, ATP carries both a phosphate and energy to various locations within a cell. When the phosphate is removed, the energy is also released. This process is result of the electron transport chain. Without the coenzyme ATP, there would be little energy available at the cellular level and normal life functions could not occur.
Here is an example of the electron transport chain. The vitamin-derived coenzyme NADH begins the process by delivering electrons. ATP is the final resulting product:





Leave a Reply
You must be logged in to post a comment.